
2.1. Three moles of an ideal gas (with temperature-independent CP = (7/2)R, CV = (5/2)R) is contained in a horizontal piston/cylinder arrangement. The piston has an area of 0.1 m2 and mass of 500 g. The initial pressure in the piston is 101 kPa. Determine the heat that must be extracted to cool the gas from 375°C to 275°C at: (a) constant pressure; (b) constant volume.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

You know the right answer?
2.1. Three moles of an ideal gas (with temperature-independent CP = (7/2)R, CV = (5/2)R) is containe...
Questions

Mathematics, 18.06.2021 01:00



Mathematics, 18.06.2021 01:00


Mathematics, 18.06.2021 01:00

History, 18.06.2021 01:00


Advanced Placement (AP), 18.06.2021 01:00


Mathematics, 18.06.2021 01:00

Mathematics, 18.06.2021 01:00


English, 18.06.2021 01:00

History, 18.06.2021 01:00


Mathematics, 18.06.2021 01:00



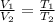 V₂ = T₂/T₁×V₁ = 548.15/648.15×0.11204 = 0.09476 m³
V₂ = T₂/T₁×V₁ = 548.15/648.15×0.11204 = 0.09476 m³

