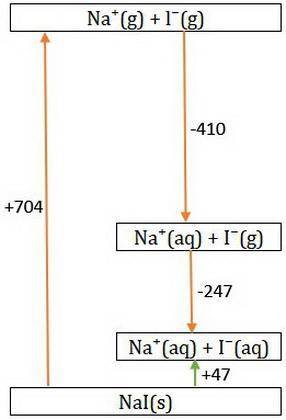
Consider Nal → Na+ + - and the following information.
Hlat = -704 kJ/mol
AHhydr of Na+=...

Chemistry, 01.03.2020 00:17 2022maldonadoleonel
Consider Nal → Na+ + - and the following information.
Hlat = -704 kJ/mol
AHhydr of Na+= -410.0 kJ/mol
AHhydr of -= -247 kJ/mol
What is the AHSol of this compound? Use AHsol = -AHlat + AHhydr.
0-867 kJ/mol
|-867.0 kJ/mol
0 47 kJ/mol
0 47.0 kJ/mol

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle
Answers: 3

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
Questions



English, 16.06.2021 08:00

Mathematics, 16.06.2021 08:00

Mathematics, 16.06.2021 08:00


English, 16.06.2021 08:00

Computers and Technology, 16.06.2021 08:00

Mathematics, 16.06.2021 08:00

Mathematics, 16.06.2021 08:00


Mathematics, 16.06.2021 08:00


Mathematics, 16.06.2021 08:00





Mathematics, 16.06.2021 08:00