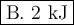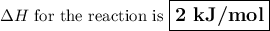Calculate the enthalpy change,,ΔH=∑ Hp - ∑ HR for the following reaction using equations 1, 2 C graphite(s) --> C diamond(s) The following is known. 1. Cgraphite(s) + O2(g) --> CO2(g) ∆H = -394 kJ 2. Cdiamond(s) + O2(g) --> CO2(g) ∆H = -396 kJ A. -763kJ B. 2kJ C. 790kJ D. -2kJ

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1


Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3
You know the right answer?
Calculate the enthalpy change,,ΔH=∑ Hp - ∑ HR for the following reaction using equations 1, 2 C grap...
Questions

Mathematics, 05.08.2019 00:50



English, 05.08.2019 00:50




Mathematics, 05.08.2019 00:50





History, 05.08.2019 00:50

Arts, 05.08.2019 00:50




Mathematics, 05.08.2019 00:50