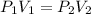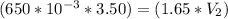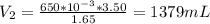Chemistry, 02.03.2020 04:06 letsgetcookingblog
A gas occupies a volume of 650.0 mL when the pressure is 3.50 atm. What will the new volume be if the pressure is reduced to 1.65 atm and the temperature remains constant.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1


Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
You know the right answer?
A gas occupies a volume of 650.0 mL when the pressure is 3.50 atm. What will the new volume be if th...
Questions

English, 25.11.2021 06:10


Physics, 25.11.2021 06:10

Mathematics, 25.11.2021 06:10




Business, 25.11.2021 06:10


Mathematics, 25.11.2021 06:10



Spanish, 25.11.2021 06:10


Chemistry, 25.11.2021 06:10