

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2


Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1

Chemistry, 23.06.2019 14:40
Uuestons niuthe no. of millimoles of hcl required to neutralize 10 ml of 0.2 m na2co3 is(a) 2.0 m mole(b) 4.0 m mole(c) 0.2 m mole(d) 0.4 m mole
Answers: 1
You know the right answer?
A molecule has the empirical formula C4H6O. If its molecular weight is determined to be about 212 g/...
Questions



Mathematics, 21.01.2020 01:31



Arts, 21.01.2020 01:31


Mathematics, 21.01.2020 01:31


Biology, 21.01.2020 01:31

Mathematics, 21.01.2020 01:31

English, 21.01.2020 01:31



Mathematics, 21.01.2020 01:31


Mathematics, 21.01.2020 01:31



Mathematics, 21.01.2020 01:31

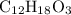 .
. 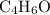 , then the molecular formula would be
, then the molecular formula would be 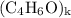 , or equivalently
, or equivalently 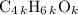 , where
, where 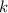 is a positive whole number (
is a positive whole number (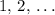 , etc.) The goal here is to find the value of
, etc.) The goal here is to find the value of 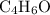 would be
would be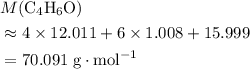 .
.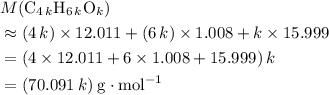 .
.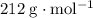 ,
, 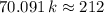 .
.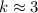 . (Round to the nearest whole number.)
. (Round to the nearest whole number.)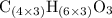 , which simplifies to
, which simplifies to 

