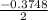Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3


Chemistry, 23.06.2019 06:30
What is the chemical formula for a compound between li and br? libr li2br libr2 libr3
Answers: 1

Chemistry, 23.06.2019 08:50
What happens after sound waves create vibrations in the ear?
Answers: 1
You know the right answer?
Calculate the number of moles of Cl2 produced at equilibrium when 3.98 mol of PCl5 is heated at 283....
Questions

Chemistry, 09.11.2019 01:31

Mathematics, 09.11.2019 01:31

Mathematics, 09.11.2019 01:31


English, 09.11.2019 01:31





Computers and Technology, 09.11.2019 01:31










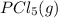 ⇄
⇄ 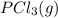 +
+ 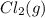
![\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0529/9897/247b7.png)
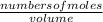
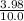
![\frac{[x][x]}{[0.398-x]}](/tpl/images/0529/9897/2afb6.png)
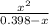
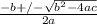
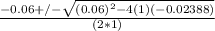
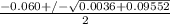
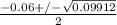
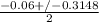
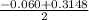 or
or 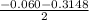
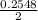 or
or