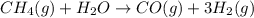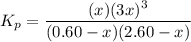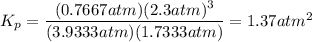Chemistry, 02.03.2020 16:51 jonestheproblem5029
Steam reforming of methane ( ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. An industrial chemist studying this reaction fills a flask with of methane gas and of water vapor at . She then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be . Calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. Round your answer to significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 03:20
What kind of intermolecular forces act between a hydrogen fluoride molecule and a hydrogen peroxide molecule? note: if there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
Answers: 1

Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 1
You know the right answer?
Steam reforming of methane ( ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrog...
Questions

Mathematics, 27.06.2019 06:00

Mathematics, 27.06.2019 06:00

History, 27.06.2019 06:00


Mathematics, 27.06.2019 06:00

Advanced Placement (AP), 27.06.2019 06:00

English, 27.06.2019 06:00

Mathematics, 27.06.2019 06:00

Mathematics, 27.06.2019 06:00



History, 27.06.2019 06:00






Mathematics, 27.06.2019 06:00