
Chemistry, 02.03.2020 16:50 jameslinimk
Calculate E o , E, and ΔG for the following cell reactions (a) Mg(s) + Sn2+(aq) ⇌ Mg2+(aq) + Sn(s) where [Mg2+] = 0.025 M and [Sn2+] = 0.040 M E o = V E = V ΔG = kJ (b) 3Zn(s) + 2Cr3+(aq) ⇌ 3Zn2+(aq) + 2Cr(s) where [Cr3+] = 0.040 M and [Zn2+] = 0.0085 M E o = V E = V ΔG = kJ

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1
You know the right answer?
Calculate E o , E, and ΔG for the following cell reactions (a) Mg(s) + Sn2+(aq) ⇌ Mg2+(aq) + Sn(s) w...
Questions


Mathematics, 10.12.2020 21:30




English, 10.12.2020 21:30

English, 10.12.2020 21:30







Mathematics, 10.12.2020 21:40

Mathematics, 10.12.2020 21:40



History, 10.12.2020 21:40

Mathematics, 10.12.2020 21:40

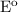 = 2.23 V, E = 2.2 V,
= 2.23 V, E = 2.2 V, 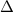 G = 424.6 kJ/mol
G = 424.6 kJ/mol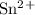 = -0.14
= -0.14 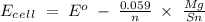

 96500
96500 



