
Chemistry, 02.03.2020 17:10 ozzypurple05
Calculate the solubility (in g/L) of CaSO 4 ( s ) CaSO4(s) in 0.400 M Na 2 SO 4 ( aq ) at 25 ° C 0.400 M Na2SO4(aq) at 25°C. The K sp Ksp of CaSO 4 CaSO4 is 4.93 × 10 − 5 4.93×10−5.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:50
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide. part a write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.
Answers: 3

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2


Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2
You know the right answer?
Calculate the solubility (in g/L) of CaSO 4 ( s ) CaSO4(s) in 0.400 M Na 2 SO 4 ( aq ) at 25 ° C 0.4...
Questions

Mathematics, 20.11.2020 19:30


History, 20.11.2020 19:30

English, 20.11.2020 19:30

Mathematics, 20.11.2020 19:30



Biology, 20.11.2020 19:30




Biology, 20.11.2020 19:30

Mathematics, 20.11.2020 19:30


Mathematics, 20.11.2020 19:30



Mathematics, 20.11.2020 19:30

History, 20.11.2020 19:30

Physics, 20.11.2020 19:30

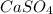 is as follows.
is as follows.
![K_{sp} = [Ca^{2+}][SO^{2-}_{4}]](/tpl/images/0530/1345/0d847.png)

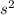
 is given as 0.4 M.
is given as 0.4 M. ![[Na_{2}SO_{4}] = [SO^{2-}_{4}]](/tpl/images/0530/1345/ebfe8.png) = 0.4 M
= 0.4 M![4.93 \times 10^{-5} = [Ca^{2+}] \times 0.4](/tpl/images/0530/1345/e5147.png)
![[Ca^{2+}]](/tpl/images/0530/1345/17576.png) = 12.325 \times 10^{-5}[/tex]
= 12.325 \times 10^{-5}[/tex]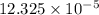 .
.


