
Chemistry, 02.03.2020 17:06 aariannahnorwoo
At a particular temperature, K = 4.1 ✕ 10−6 for the following reaction. 2 CO2(g) 2 CO(g) + O2(g) If 2.3 moles of CO2 is initially placed into a 4.9-L vessel, calculate the equilibrium concentrations of all species.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
You know the right answer?
At a particular temperature, K = 4.1 ✕ 10−6 for the following reaction. 2 CO2(g) 2 CO(g) + O2(g) If...
Questions

Mathematics, 13.11.2020 19:40


Computers and Technology, 13.11.2020 19:40

Biology, 13.11.2020 19:40

Biology, 13.11.2020 19:40

Mathematics, 13.11.2020 19:40


Mathematics, 13.11.2020 19:40

Arts, 13.11.2020 19:40







Advanced Placement (AP), 13.11.2020 19:40




Mathematics, 13.11.2020 19:40

![[O_2]](/tpl/images/0530/1146/b0db0.png) = 0.0124 = 12.4 ×10⁻³ M
= 0.0124 = 12.4 ×10⁻³ M![[CO]](/tpl/images/0530/1146/32558.png) = 0.0248 = 2.48 ×10⁻² M
= 0.0248 = 2.48 ×10⁻² M![[CO_2]](/tpl/images/0530/1146/5494d.png) = 0.4442 M
= 0.4442 M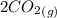 ⇄
⇄ 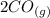 +
+ 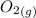
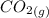 =
= 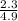 = 0.469
= 0.469![\frac{[CO]^2[O]}{[CO_2]^2}](/tpl/images/0530/1146/9940b.png)
![\frac{[2x]^2[x]}{[0.469-2x]^2}](/tpl/images/0530/1146/25de6.png)
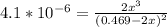
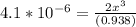
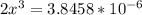
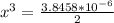
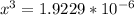
![x=\sqrt[3]{1.9929*10^{-6}}](/tpl/images/0530/1146/6ad64.png)


