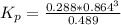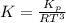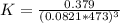Chemistry, 02.03.2020 16:54 cristinanina
Consider the decomposition of the compound C5H6O3 as follows below. C5H6O3(g) → C2H6(g) + 3 CO(g) When a 5.63-g sample of pure C5H6O3(g) was sealed into an otherwise empty 2.50 L flask and heated to 200.°C, the pressure in the flask gradually rose to 1.63 atm and remained at that value. Calculate K for this reaction.

Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1

Chemistry, 23.06.2019 12:30
Choose one literary selection from this semester in which you think the setting has a great impact on the work. in a full paragraph name the work, describe the setting, and explain why it is so important to the overall story or poem.
Answers: 1
You know the right answer?
Consider the decomposition of the compound C5H6O3 as follows below. C5H6O3(g) → C2H6(g) + 3 CO(g) Wh...
Questions

History, 26.09.2019 05:00

Social Studies, 26.09.2019 05:00

History, 26.09.2019 05:00

History, 26.09.2019 05:00

Mathematics, 26.09.2019 05:00


Mathematics, 26.09.2019 05:00

History, 26.09.2019 05:00

History, 26.09.2019 05:00


Chemistry, 26.09.2019 05:00

Mathematics, 26.09.2019 05:00

Advanced Placement (AP), 26.09.2019 05:00

History, 26.09.2019 05:00



Mathematics, 26.09.2019 05:00



Mathematics, 26.09.2019 05:00