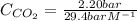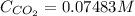Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 23.06.2019 04:31
Which molecules are more strongly attracted to one another -c3h8o molecules that make up liquid rubbing alcohol or ch4 molecules that make up methane gas
Answers: 3

Chemistry, 23.06.2019 07:30
To separate a mixture of hard candies nd marbles the most efficient method would be
Answers: 3
You know the right answer?
The Henry's law constant for CO 2 ( g ) CO2(g) in water at 25 ∘ C 25 ∘C is 29.4 bar⋅M − 1 29.4 bar·M...
Questions



Mathematics, 09.01.2020 03:31

Mathematics, 09.01.2020 03:31

Social Studies, 09.01.2020 03:31







Mathematics, 09.01.2020 03:31

Mathematics, 09.01.2020 03:31


Mathematics, 09.01.2020 03:31

French, 09.01.2020 03:31


Mathematics, 09.01.2020 03:31


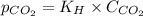
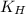 = Henry's constant =
= Henry's constant = 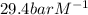
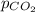 = partial pressure of carbonated drink = 2.20 bar
= partial pressure of carbonated drink = 2.20 bar