
Chemistry, 02.03.2020 17:16 genyjoannerubiera
For alpha decay to cause a loss in mass, such as that in the uranium 238 conversion to thorium 234, what must the atomic weight of the alpha particle be?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1


Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
For alpha decay to cause a loss in mass, such as that in the uranium 238 conversion to thorium 234,...
Questions

Physics, 07.04.2020 06:33

Social Studies, 07.04.2020 06:33


Chemistry, 07.04.2020 06:33


Mathematics, 07.04.2020 06:33


Mathematics, 07.04.2020 06:33

Spanish, 07.04.2020 06:33

English, 07.04.2020 06:33

History, 07.04.2020 06:34

English, 07.04.2020 06:34


Geography, 07.04.2020 06:35




Biology, 07.04.2020 06:35


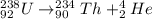
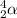 .
.

