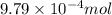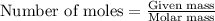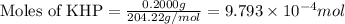Chemistry, 02.03.2020 17:28 janreyes39
As part of Lab 4 you will make and standardize a solution of NaOH(aq). Suppose in the lab you measure the solid NaOH and dissolve it into 100.0 mL of water. You then measure 0.2000 g of KHP (204.22 g/mol) and place it in a clean, dry 100-mL beaker, and then dissolve the KHP in about 25 mL of water and add a couple of drops of phenolphthalein indicator. You titrate this with your NaOH(aq) solution and find that the titration requires 9.78 mL of NaOH(aq). Part 1: How many moles of KHP are in your sample

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1


Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
You know the right answer?
As part of Lab 4 you will make and standardize a solution of NaOH(aq). Suppose in the lab you measur...
Questions



History, 14.06.2021 22:40

Mathematics, 14.06.2021 22:40









Mathematics, 14.06.2021 22:40

Business, 14.06.2021 22:40


Mathematics, 14.06.2021 22:40

Mathematics, 14.06.2021 22:40

Mathematics, 14.06.2021 22:40


Mathematics, 14.06.2021 22:40