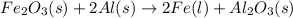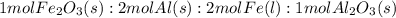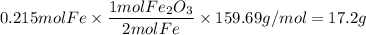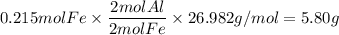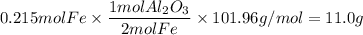Over the years, the thermite reaction has been used for welding railroad rails, in incendiary bombs, and to ignite solid-fuel rocket motors. The reaction is given below. Fe2O3(s) + 2 Al(s) 2 Fe(l) + Al2O3(s) What masses of iron(III) oxide and aluminum must be used to produce 12.0 g iron? iron (III) oxide WebAssign will check your answer for the correct number of significant figures. 17.2 Correct: Your answer is correct. g aluminum WebAssign will check your answer for the correct number of significant figures. 2.98 Incorrect: Your answer is incorrect. g What is the maximum mass of aluminum oxide that could be produced?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 23.06.2019 01:00
What two factors can affect the properties of a hydrocarbon? a. the number of its carbon atoms and the number of single bonds b. the number of its carbon atoms and the arrangement of its atoms c. the arrangement of its atoms and the number of its double bonds
Answers: 1
You know the right answer?
Over the years, the thermite reaction has been used for welding railroad rails, in incendiary bombs,...
Questions

Chemistry, 22.05.2020 03:57

Mathematics, 22.05.2020 03:57

Mathematics, 22.05.2020 03:57

Mathematics, 22.05.2020 03:57




Mathematics, 22.05.2020 03:57

Mathematics, 22.05.2020 03:57



Mathematics, 22.05.2020 03:57

Mathematics, 22.05.2020 03:57



Biology, 22.05.2020 03:57



History, 22.05.2020 03:57