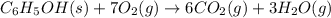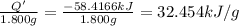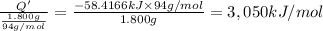A 1.800-g sample of solid phenol (C6H5OH(s)) was burned in a bomb calorimeter whose total heat capacity is 11.66 kJ/?C. The temperature of the calorimeter plus contents increased from 21.36?Cto 26.37?C. Part A
Write a balanced chemical equation for the bomb calorimeter reaction.
Part B:
What is the heat of combustion per gram of phenol?Part C:
Per mole of phenol?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

You know the right answer?
A 1.800-g sample of solid phenol (C6H5OH(s)) was burned in a bomb calorimeter whose total heat capac...
Questions

Mathematics, 12.12.2021 02:40


Mathematics, 12.12.2021 02:40

Chemistry, 12.12.2021 02:50


Chemistry, 12.12.2021 02:50

German, 12.12.2021 02:50

Mathematics, 12.12.2021 02:50



History, 12.12.2021 02:50


Computers and Technology, 12.12.2021 02:50


Arts, 12.12.2021 02:50





Mathematics, 12.12.2021 02:50