
Chemistry, 02.03.2020 18:18 cwsmith8026
. When iron rusts in air, iron(III) oxide is produced. How many moles of oxygen react with 2.4 mol of iron in the rusting reaction? 4Fe( s ) + 3O 2 ( g ) → 2Fe 2 O 3 ( s )

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3


Chemistry, 23.06.2019 09:00
Astream of surface water reaches a porous portion of sediment and seeps into the ground. this water eventually joins a large reservoir of water located beneath the earth's surface. the example above describes the interacting with the a. cryosphere; biosphere b. hydrosphere; biosphere c. hydrosphere; geosphere d. cryosphere; geosphere
Answers: 3
You know the right answer?
. When iron rusts in air, iron(III) oxide is produced. How many moles of oxygen react with 2.4 mol o...
Questions




Mathematics, 11.03.2021 01:00

History, 11.03.2021 01:00




Chemistry, 11.03.2021 01:00


Mathematics, 11.03.2021 01:00

Mathematics, 11.03.2021 01:00



Physics, 11.03.2021 01:00

Mathematics, 11.03.2021 01:00




Mathematics, 11.03.2021 01:00

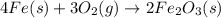
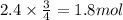 of oxygen gas.
of oxygen gas.

