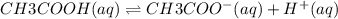Chemistry, 02.03.2020 18:17 biancaTomas010B
For each of the following reactions, write the appropriate equilibrium equation. (Equilibrium expressions take the general form: Keq = [C]c / [A]a . [B]b. Subscripts and superscripts that include letters must be enclosed in braces {}.) (a) CH3COOH(aq) equilibrium reaction arrow CH3COO−(aq) + H +(aq)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 23.06.2019 02:50
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2

Chemistry, 23.06.2019 13:30
Why hydrochloric acid neutralized first when you titrate a mixture of hcl& ch3cooh against standard sodium hydroxide
Answers: 1

Chemistry, 23.06.2019 15:30
Sodium chloride can be made as follows: 2na + cl2 ? 2nacl i calculate the maximum amount of nacl possible if 2.3 g of sodium was reacted with excess chlorine. show all your workings.
Answers: 3
You know the right answer?
For each of the following reactions, write the appropriate equilibrium equation. (Equilibrium expres...
Questions

Biology, 27.07.2019 01:40

History, 27.07.2019 01:40

History, 27.07.2019 01:40

Biology, 27.07.2019 01:40

Social Studies, 27.07.2019 01:40



History, 27.07.2019 01:40

Social Studies, 27.07.2019 01:40




Mathematics, 27.07.2019 01:40



Geography, 27.07.2019 01:40


Health, 27.07.2019 01:40


Biology, 27.07.2019 01:40

![K_{eq}=\frac{[CH_COO^-]^1[H^+]^1}{[CH_3COOH]^1}](/tpl/images/0530/3152/7d0bc.png)
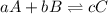
![K_{eq}=\frac{[C]^c}{[A]^a[B]^b}](/tpl/images/0530/3152/98de9.png)