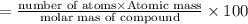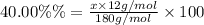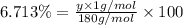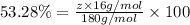Chemistry, 02.03.2020 18:30 leylaanderson85311
A compound consists of 40.00% C, 6.713% H and 53.28% O on a mass basis and has a molar mass of about 180 g/mole. Determine the molecular formula of the compound.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1
You know the right answer?
A compound consists of 40.00% C, 6.713% H and 53.28% O on a mass basis and has a molar mass of about...
Questions


Mathematics, 01.09.2021 07:40

Biology, 01.09.2021 07:40

Mathematics, 01.09.2021 07:40


Mathematics, 01.09.2021 07:40

Social Studies, 01.09.2021 07:40





Mathematics, 01.09.2021 07:40


Mathematics, 01.09.2021 07:40

Biology, 01.09.2021 07:40



Mathematics, 01.09.2021 07:40

History, 01.09.2021 07:40

Biology, 01.09.2021 07:40

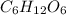 .
.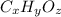 .in
.in