
Chemistry, 02.03.2020 19:06 lizzie3545
Calculate the mass of H2OH2O produced by metabolism of 2.4 kgkg of fat, assuming the fat consists entirely of tristearin (C57H110O6C57H110O6), a typical animal fat, and assuming that during metabolism, tristearin reacts with O2O2 to form only CO2CO2 and H2OH2O.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:00
The study of witch tree monkeys feed in is part of the science life
Answers: 1

Chemistry, 21.06.2019 16:00
Measuring which physical property is most likely to produce the most precise results when trying to identify a substance
Answers: 1

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2
You know the right answer?
Calculate the mass of H2OH2O produced by metabolism of 2.4 kgkg of fat, assuming the fat consists en...
Questions

Medicine, 16.10.2020 18:01

Biology, 16.10.2020 18:01




Mathematics, 16.10.2020 18:01

History, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01




Geography, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01

Chemistry, 16.10.2020 18:01

Physics, 16.10.2020 18:01

Biology, 16.10.2020 18:01


Mathematics, 16.10.2020 18:01


History, 16.10.2020 18:01

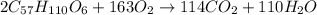
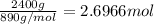
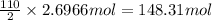 of water
of water

