c5h12(g)+8o2(g)→5co2(g)+6h2o(g)

Chemistry, 15.10.2019 10:10 ayowazzzgood
The combustion of pentane, c5h12, occurs via the reaction
c5h12(g)+8o2(g)→5co2(g)+6h2o(g)
with heat of formation values given by the following table:
substance δh∘f
(kj/mol)
c5h12 (g) -119.9
co2(g) −393.5
h2o(g) −241.8
calculate the enthalpy for the combustion of 1 mole of pentane.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1
You know the right answer?
The combustion of pentane, c5h12, occurs via the reaction
c5h12(g)+8o2(g)→5co2(g)+6h2o(g)
c5h12(g)+8o2(g)→5co2(g)+6h2o(g)
Questions




Mathematics, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01


Social Studies, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01

History, 28.09.2020 14:01


Mathematics, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01


Mathematics, 28.09.2020 14:01


History, 28.09.2020 14:01




Mathematics, 28.09.2020 14:01

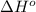
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0321/5182/45485.png)
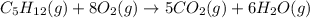
![\Delta H^o_{rxn}=[(n_{(CO_2)}\times \Delta H^o_f_{(CO_2)})+(n_{(H_2O)}\times \Delta H^o_f_{(H_2O)})]-[(n_{(C_5H_{12})}\times \Delta H^o_f_{(C_5H_{12})})+(n_{(O_2)}\times \Delta H^o_f_{(O_2)})]](/tpl/images/0321/5182/dfc82.png)
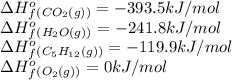
![\Delta H^o_{rxn}=[(5\times -393.5)+(6\times -241.8)]-[(1\times -393.5)+(8\times 0)=-3024.8kJ](/tpl/images/0321/5182/8ec80.png)


