
Chemistry, 02.03.2020 19:12 chandranewlon
The student determines the molar mass of the gas to be 64 g mol-1. write the expression (set-up) for calculating the percent error in the experimental value, assuming that the unknown gas is butane (molar mass 58 g mol-1). calculations are not required

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2

Chemistry, 23.06.2019 01:00
Which of the following is a physical change? a.burning a piece of wood b.sawing a piece of wood in half c.rust forming on an iron fence d.a copper roof changing color from orange to green
Answers: 1

Chemistry, 23.06.2019 06:30
Which of the following steps is not likely to take place during cellular respiration? (5 points) select one: a. oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. c. simple sugar breaks down. d. energy is used up.
Answers: 1
You know the right answer?
The student determines the molar mass of the gas to be 64 g mol-1. write the expression (set-up) for...
Questions


Mathematics, 29.04.2021 18:50



Mathematics, 29.04.2021 18:50

Mathematics, 29.04.2021 18:50



Business, 29.04.2021 18:50




Mathematics, 29.04.2021 18:50

Social Studies, 29.04.2021 18:50

Mathematics, 29.04.2021 18:50


Arts, 29.04.2021 18:50



Mathematics, 29.04.2021 18:50

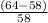 x 100%
x 100%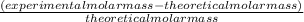 x 100%
x 100%

