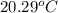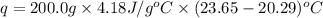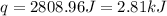A 100.0 mL of 0.500 M HBr at 20.29 oC is added to 100.0 mL of 0.500 M KOH (also at 20.29 oC). After mixing, the temperature rises to 23.65 oC. Calculate the heat of this reaction. [assuming the density and specific heat of HBr and KOH is the same as water, 1.0 g/mL; 4.18 J/g oC, and the volume of the solution is additive].

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1

Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1
You know the right answer?
A 100.0 mL of 0.500 M HBr at 20.29 oC is added to 100.0 mL of 0.500 M KOH (also at 20.29 oC). After...
Questions

History, 04.02.2021 23:30







Biology, 04.02.2021 23:30

Social Studies, 04.02.2021 23:30



Mathematics, 04.02.2021 23:30

Mathematics, 04.02.2021 23:30

Mathematics, 04.02.2021 23:30

English, 04.02.2021 23:30

Mathematics, 04.02.2021 23:30



Mathematics, 04.02.2021 23:30

Chemistry, 04.02.2021 23:30

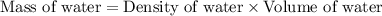
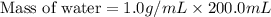
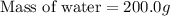
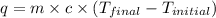
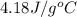
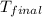 = final temperature =
= final temperature = 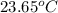
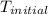 = initial temperature =
= initial temperature =