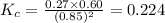Chemistry, 02.03.2020 21:19 braxtengames
Calculate Kc for the reaction: 2 HI(g) ⇄ H2(g) + I2(g) given that the concentrations of each species at equilibrium are as follows: [HI] = 0.85 mol/L, [I2] = 0.60 mol/L, [H2] = 0.27 mol/L.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3
You know the right answer?
Calculate Kc for the reaction: 2 HI(g) ⇄ H2(g) + I2(g) given that the concentrations of each species...
Questions



Mathematics, 05.09.2020 23:01

History, 05.09.2020 23:01








Mathematics, 05.09.2020 23:01


Computers and Technology, 05.09.2020 23:01


English, 05.09.2020 23:01

Mathematics, 05.09.2020 23:01


Mathematics, 05.09.2020 23:01

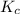 for the given reaction is 0.224
for the given reaction is 0.224 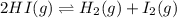
![K_c=\frac{[H_2][I_2]}{[HI]^2}](/tpl/images/0530/7257/ef85e.png)
![[HI]_{eq}=0.85M](/tpl/images/0530/7257/9952a.png)
![[H_2]_{eq}=0.27M](/tpl/images/0530/7257/01e4f.png)
![[I_2]_{eq}=0.60M](/tpl/images/0530/7257/de9f5.png)