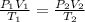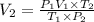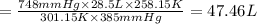Chemistry, 28.11.2019 12:31 destinyhope4776
Aweather balloon is inflated to a volume of 28.5 l at a pressure of 748 mmhg and a temperature of 28.0 degrees c. the balloon rises in the atmosphere to an altitude of 25,000 feet, where the pressure is 385 mmhg and the temperature is -15.0 degrees c. assuming the balloon can freely expand, calculate the volume of the balloon at this altitude.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Urea, co(nh2)2, is manufactured on a large scale for use in producing urea-formaldehyde plastics and as a fertilizer. what is the maximum mass of urea that can be manufactured from the co2 produced by combustion of 1.00 x 104 grams of co2?
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1
You know the right answer?
Aweather balloon is inflated to a volume of 28.5 l at a pressure of 748 mmhg and a temperature of 28...
Questions




Mathematics, 12.05.2021 21:30




Mathematics, 12.05.2021 21:30


SAT, 12.05.2021 21:30


Mathematics, 12.05.2021 21:30

Mathematics, 12.05.2021 21:30


Mathematics, 12.05.2021 21:30

Mathematics, 12.05.2021 21:30


Mathematics, 12.05.2021 21:30


Mathematics, 12.05.2021 21:30

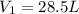
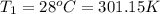
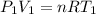 ...[1]
...[1]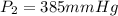
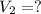
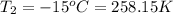
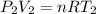 ...[2]
...[2]