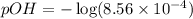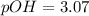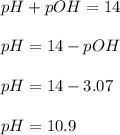Chemistry, 02.03.2020 20:56 eemorales5100
Calculate the pH and pOH of the solutions with the following hydrogen ion or hydroxide ion concentrations. Indicate which solutions are acidic, basic, or neutral. (And please show how to solve! Thanks!) a. [H+]= 3.45x10^-8 M b. [H+]= 2.0x10^-5 M c. [H+]= 7.0x10^-8 M d. [OH-]= 8.56x10^-4 M

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 23.06.2019 06:20
What is the magnitude of the force of gravity between to 1000 kg cars which are separated by distance of 25. 0 km on an interstate highway? the force between the two cars will be what
Answers: 3
You know the right answer?
Calculate the pH and pOH of the solutions with the following hydrogen ion or hydroxide ion concentra...
Questions

















Geography, 10.03.2020 18:30


Mathematics, 10.03.2020 18:30

![pH=-\log [H^+]](/tpl/images/0530/6113/37e81.png)
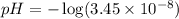
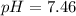
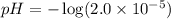
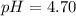
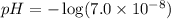
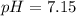
![pOH=-\log [OH^-]](/tpl/images/0530/6113/1fac1.png)