
Chemistry, 02.03.2020 21:07 jahnoibenjamin
Which of the following solutions would have the highest [H3O+]? Assume that they are all 0.10 M in acid at 25∘C. The acid is followed by its Ka value. C) HNO2, 4.6 × 10-4 B) HCN, 4.9 × 10-10 E) HClO2, 1.1 × 10-2 A) HF, 3.5 × 10-4 D) HCHO2, 1.8 × 10-4

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
Which of the following solutions would have the highest [H3O+]? Assume that they are all 0.10 M in a...
Questions

Mathematics, 19.08.2019 17:00


Social Studies, 19.08.2019 17:00

Mathematics, 19.08.2019 17:00


History, 19.08.2019 17:00


English, 19.08.2019 17:00


Computers and Technology, 19.08.2019 17:00

History, 19.08.2019 17:00


Mathematics, 19.08.2019 17:00


Social Studies, 19.08.2019 17:00

Mathematics, 19.08.2019 17:00

Chemistry, 19.08.2019 17:00

Mathematics, 19.08.2019 17:00

Computers and Technology, 19.08.2019 17:00

History, 19.08.2019 17:00

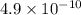
![[H^+]=\sqrt{(K_aC)}](/tpl/images/0530/6657/2da9a.png)
![pH=-\log [H^+]](/tpl/images/0530/6657/37e81.png)
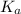 (dissociation constant) increases then the value of hydrogen ion concentration also increases and the value of pH decreases.
(dissociation constant) increases then the value of hydrogen ion concentration also increases and the value of pH decreases.

