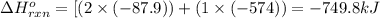Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1


Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
Consider the following thermochemical equations.
PCl5 (s)→PCl3 (g)+Cl2 (g) ΔH∘rxn= 87.9k...
PCl5 (s)→PCl3 (g)+Cl2 (g) ΔH∘rxn= 87.9k...
Questions

Business, 05.04.2020 04:59



Mathematics, 05.04.2020 04:59







English, 05.04.2020 04:59

Biology, 05.04.2020 04:59



History, 05.04.2020 04:59




Chemistry, 05.04.2020 04:59

Mathematics, 05.04.2020 04:59

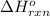 for the reaction is -749.8 kJ.
for the reaction is -749.8 kJ.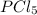 follows:
follows: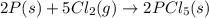
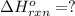
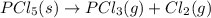
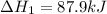 ( × 2)
( × 2)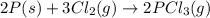
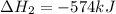
![\Delta H^o_{rxn}=[2\times (-\Delta H_1)]+[1\times \Delta H_2]](/tpl/images/0530/6491/d8429.png)