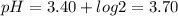Chemistry, 02.03.2020 21:50 harcharikc8275
A weak acid with a Ka of 4.0 x 10-4 is mixed with its sodium salt to form a solution that contains twice as many moles of the sodium salt than of the weak acid. What is the pH of the solution?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1

Chemistry, 23.06.2019 14:00
How is the electron sea model of metallic bonding different from the band theory? how are they the same? give at least one similarity and one difference between the models
Answers: 2

Chemistry, 23.06.2019 14:00
Which statement describes the arrhenius interpretation of acids and bases?
Answers: 1
You know the right answer?
A weak acid with a Ka of 4.0 x 10-4 is mixed with its sodium salt to form a solution that contains t...
Questions



English, 04.04.2020 11:06











Mathematics, 04.04.2020 11:07

Engineering, 04.04.2020 11:07

Mathematics, 04.04.2020 11:07




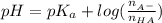
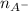 and
and 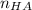 represent number of moles of
represent number of moles of 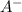 ion and HA respectively.
ion and HA respectively.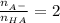 and
and