
Chemistry, 02.03.2020 22:42 shelbycg02
When 1.00 g of thiamine hydrochloride (also called vitamin B1 hydrochloride) is dissolved in water, then diluted to 10.00 mL, the pH of the resulting solution is 4.50. The formula weight of thiamine hydrochloride is 337.3 g/mol. Calculate Ka.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

You know the right answer?
When 1.00 g of thiamine hydrochloride (also called vitamin B1 hydrochloride) is dissolved in water,...
Questions

English, 11.09.2019 22:30



Mathematics, 11.09.2019 22:30

Mathematics, 11.09.2019 22:30

Mathematics, 11.09.2019 22:30



Mathematics, 11.09.2019 22:30

English, 11.09.2019 22:30


English, 11.09.2019 22:30

Mathematics, 11.09.2019 22:30




Mathematics, 11.09.2019 22:30

Medicine, 11.09.2019 22:30


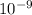
![\frac{[H+] [A-]}{[HA]}](/tpl/images/0530/9044/5a54d.png)
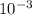

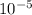
![\frac{[0.0000316] [0.000316]}{0.296}](/tpl/images/0530/9044/484a7.png)


