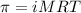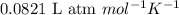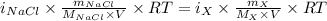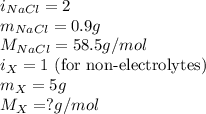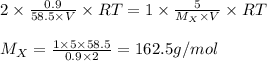Chemistry, 02.03.2020 23:07 bryanmcmillianjr
The osmotic pressure of a 0.9 weight percent solution of sodium chloride (NaCl) is equal to the osmotic pressure of a 5 weight percent solution of non-dissociating molecule X. What is the molecular weight of molecule X

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

You know the right answer?
The osmotic pressure of a 0.9 weight percent solution of sodium chloride (NaCl) is equal to the osmo...
Questions



History, 26.03.2021 22:20


Chemistry, 26.03.2021 22:20



Mathematics, 26.03.2021 22:20

Mathematics, 26.03.2021 22:20

Mathematics, 26.03.2021 22:20

Mathematics, 26.03.2021 22:20

Mathematics, 26.03.2021 22:20

Mathematics, 26.03.2021 22:20

History, 26.03.2021 22:20

Mathematics, 26.03.2021 22:20

Mathematics, 26.03.2021 22:20


Biology, 26.03.2021 22:20

Mathematics, 26.03.2021 22:20