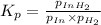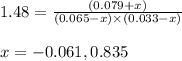Chemistry, 02.03.2020 23:05 shukriabdisabrie
Gaseous indium dihydride is formed from the elements at elevated temperature:
ln(g)+H2(g)?lnH2(g)Kp = 1.48 at 973 K
Partial pressures measured in a reaction vessel are:
PIn = 0.0650atm , PH2 = 0.0330atm , PInH2 = 0.0790atm
1. Calculate Qp
2. Determine the direction of reaction to attain equilibrium
3. Determine the equilibrium partial pressure of In
4. Determine the equilibrium partial pressure of H2
5. Determine the equilibrium partial pressure of InH2

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1


Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
You know the right answer?
Gaseous indium dihydride is formed from the elements at elevated temperature:
ln(g)+H2(g...
ln(g)+H2(g...
Questions


Computers and Technology, 25.09.2020 14:01

Mathematics, 25.09.2020 14:01



Mathematics, 25.09.2020 14:01

English, 25.09.2020 14:01


Mathematics, 25.09.2020 14:01



Mathematics, 25.09.2020 14:01

Mathematics, 25.09.2020 14:01



Mathematics, 25.09.2020 14:01


Mathematics, 25.09.2020 14:01

English, 25.09.2020 14:01

Mathematics, 25.09.2020 14:01

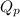 for above reaction is 36.83
for above reaction is 36.83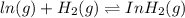
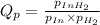
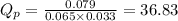
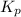 of the reaction = 1.48
of the reaction = 1.48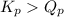 ; the reaction is product favored.When
; the reaction is product favored.When 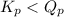 ; the reaction is reactant favored.When
; the reaction is reactant favored.When 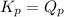 ; the reaction is in equilibrium.
; the reaction is in equilibrium.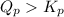 for the given reaction, the reaction is reactant favored.
for the given reaction, the reaction is reactant favored.