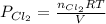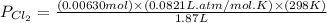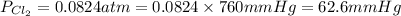Chemistry, 02.03.2020 23:03 angeleyes4u610p6np54
A 1.87 L reaction vessel, initially at 298 K, contains chlorine gas at a partial pressure of 337 mmHg and fluorine gas at a partial pressure of 820 mmHg . What is the partial pressure of excess reactant after the reaction occurs as completely as possible

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 23.06.2019 04:00
Silver reacts with oxygen to produce silver oxide. (write balanced chemical equation and identify type of chemical reaction.)
Answers: 1
You know the right answer?
A 1.87 L reaction vessel, initially at 298 K, contains chlorine gas at a partial pressure of 337 mmH...
Questions



English, 10.11.2020 06:20

Social Studies, 10.11.2020 06:20



Mathematics, 10.11.2020 06:20

Business, 10.11.2020 06:20

Chemistry, 10.11.2020 06:20

Computers and Technology, 10.11.2020 06:20

Mathematics, 10.11.2020 06:20


Mathematics, 10.11.2020 06:20

Business, 10.11.2020 06:20



Mathematics, 10.11.2020 06:30

Health, 10.11.2020 06:30

Mathematics, 10.11.2020 06:30

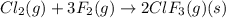
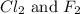
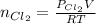
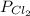 = partial pressure of
= partial pressure of 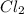 = 337 mmHg = 0.443 atm
= 337 mmHg = 0.443 atm 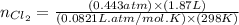
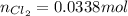
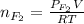
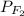 = partial pressure of
= partial pressure of 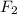 = 820 mmHg = 1.08 atm
= 820 mmHg = 1.08 atm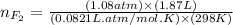
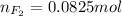
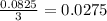 moles of
moles of