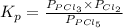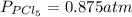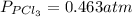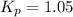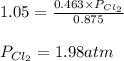Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1
You know the right answer?
The equilibrium constant for the decomposition of PCl5 at 250 celcius is 1.05. PCl5(g)-->PCl3(g)+...
Questions

History, 11.11.2020 23:50




Mathematics, 11.11.2020 23:50

Computers and Technology, 11.11.2020 23:50

Mathematics, 11.11.2020 23:50



Mathematics, 11.11.2020 23:50

Mathematics, 11.11.2020 23:50

Social Studies, 11.11.2020 23:50

Mathematics, 11.11.2020 23:50

Chemistry, 11.11.2020 23:50

Biology, 11.11.2020 23:50



Health, 11.11.2020 23:50


Mathematics, 11.11.2020 23:50

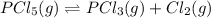
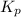 for above reaction follows:
for above reaction follows: