

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Asample of gas occupies 17 ml at –112°c. what volume does the sample occupy at 70°c a. 10.6 ml b. 27 ml c. 36 ml d. 8.0 ml you
Answers: 1

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1

Chemistry, 23.06.2019 09:00
The vapor pressure of water at 25.0°c is 23.8 torr. determine the mass of glucose (molar mass = 180 g/mol) needed to add to 500.0 g of water to change the vapor pressure to 22.8 torr.
Answers: 1
You know the right answer?
At 25 °C, an aqueous solution has an equilibrium concentration of 0.00423 0.00423 M for a generic ca...
Questions


Biology, 02.10.2019 05:00



Law, 02.10.2019 05:00








Business, 02.10.2019 05:00


History, 02.10.2019 05:00


History, 02.10.2019 05:00


Mathematics, 02.10.2019 05:00

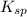 of the generic salt AB is,
of the generic salt AB is, 
 = 0.00423 M
= 0.00423 M = 0.00423 M
= 0.00423 M
![K_{sp}=[A^+][B^-]](/tpl/images/0531/1161/7fe85.png)




