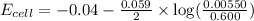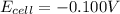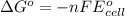Chemistry, 03.03.2020 00:06 cheyennebatz3609
Calculate the emf for the following reaction. Will the reaction occur spontaneously at 25°C, given that [Fe2+] = 0.600 M and [Cd2+] = 0.00550 M? Cd(s) + Fe2+(aq)→Cd2+(aq) + Fe(s) E o Cd2+/Cd = −0.40 V E o Fe2+/Fe = −0.44 V

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 23.06.2019 07:00
Which of the following statements is true? an atom consists of protons, electrons, and neutrons.an atom consists of protons and neutrons.an atom consists of electrons bonded to one another.an atom consists of protons bonded to one another.
Answers: 1

Chemistry, 23.06.2019 09:10
A155.0 9 piece of copper at 182 °c is dropped into 2500 g of water at 23.9 °c. (the specific heat of copper is 0.385 1/9°c.) calculate the final temperature of the mixture. (assume no heat loss to the surroundings.)
Answers: 2
You know the right answer?
Calculate the emf for the following reaction. Will the reaction occur spontaneously at 25°C, given t...
Questions


Social Studies, 25.11.2021 05:30




Spanish, 25.11.2021 05:30



English, 25.11.2021 05:30

Mathematics, 25.11.2021 05:30

Mathematics, 25.11.2021 05:30


English, 25.11.2021 05:30


Health, 25.11.2021 05:30

Computers and Technology, 25.11.2021 05:30



Mathematics, 25.11.2021 05:30

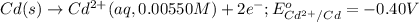
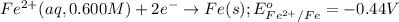
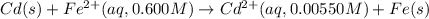
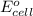 of the reaction, we use the equation:
of the reaction, we use the equation: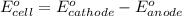
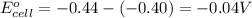
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Cd^{2+}]}{[Fe^{2+}]}](/tpl/images/0531/1947/ee65e.png)
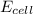 = electrode potential of the cell = ?
= electrode potential of the cell = ?![[Cd^{2+}]=0.00550M](/tpl/images/0531/1947/3e0ad.png)
![[Fe^{2+}]=0.600M](/tpl/images/0531/1947/5e50b.png)