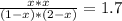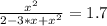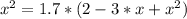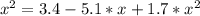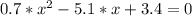Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 22.06.2019 21:00
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1
You know the right answer?
The reversible chemical reaction A+B⇌C+D has the following equilibrium constant: Kc=[C][D][A][B]=1.7...
Questions

Mathematics, 09.11.2019 21:31






Mathematics, 09.11.2019 21:31

Mathematics, 09.11.2019 21:31




Biology, 09.11.2019 21:31


Mathematics, 09.11.2019 21:31

Biology, 09.11.2019 21:31

History, 09.11.2019 21:31

History, 09.11.2019 21:31



English, 09.11.2019 21:31

![Kc=\frac{[C]^{c} *[D]^{d} }{[A]^{a} *[B]^{b} }](/tpl/images/0531/2647/eda24.png)
![Kc=\frac{[C]*[D]}{[A]*[B]}=1.7](/tpl/images/0531/2647/e4842.png)