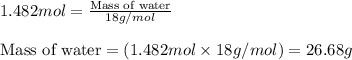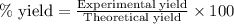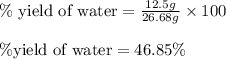Chemistry, 03.03.2020 00:53 jennaranelli05
Aqueous sulfuric acid reacts with solid sodium hydroxide to produce aqueous sodium sulfate and liquid water . If of water is produced from the reaction of of sulfuric acid and of sodium hydroxide, calculate the percent yield of water.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 23.06.2019 09:00
Agust of wind blowing east pushes against a ball. when will the wind do work on the ball? when the ball moves to the east when the ball moves to the north when the ball stays in one place when the ball moves north or south
Answers: 1
You know the right answer?
Aqueous sulfuric acid reacts with solid sodium hydroxide to produce aqueous sodium sulfate and liqui...
Questions


History, 30.07.2019 15:00





History, 30.07.2019 15:00


Biology, 30.07.2019 15:10

Biology, 30.07.2019 15:10

English, 30.07.2019 15:10




English, 30.07.2019 15:10

Biology, 30.07.2019 15:10



Mathematics, 30.07.2019 15:10

English, 30.07.2019 15:10

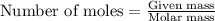 .....(1)
.....(1)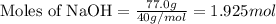
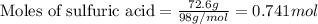
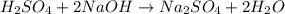
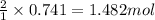 of NaOH
of NaOH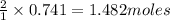 of water
of water