
Consider the following three processes: (1) Melting of ice at room temperature (2) Boiling of water at 101°C (3) Dissolving of NH4NO3 with water in an instant cold pack? Which statement(s) that are true about ALL three of the given processes? (Choose one or more)(A) Endothermic(B) Exothermic(C) Nonspontaneous(D) Spontaneous(E) none of these statements can be used to describe all three processes

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1
You know the right answer?
Consider the following three processes: (1) Melting of ice at room temperature (2) Boiling of water...
Questions

Mathematics, 19.10.2021 20:20

Physics, 19.10.2021 20:20


Mathematics, 19.10.2021 20:20


Chemistry, 19.10.2021 20:20

English, 19.10.2021 20:20



Mathematics, 19.10.2021 20:20

French, 19.10.2021 20:20


English, 19.10.2021 20:20


Social Studies, 19.10.2021 20:20


Computers and Technology, 19.10.2021 20:20

Mathematics, 19.10.2021 20:20

English, 19.10.2021 20:20

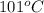 is also an endothermic process. This is because heat is absorbed by water molecules due to which their state changes from liquid to vapor form. When
is also an endothermic process. This is because heat is absorbed by water molecules due to which their state changes from liquid to vapor form. When 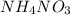 is dissolved in water then heat is absorbed as there occurs a decrease in temperature of water. Hence, it is also an endothermic reaction.
is dissolved in water then heat is absorbed as there occurs a decrease in temperature of water. Hence, it is also an endothermic reaction.

