
Chemistry, 03.03.2020 03:20 jazzlashayy1487
A 30.0-mL sample of an unknown strong base is neutralized after the addition of 12.0 mL of a 0.150 M HNO3 solution. If the unknown base concentration is 0.0300 M, give some possible identities for the unknown base.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1
You know the right answer?
A 30.0-mL sample of an unknown strong base is neutralized after the addition of 12.0 mL of a 0.150 M...
Questions

Mathematics, 25.04.2021 22:10

Mathematics, 25.04.2021 22:10

Mathematics, 25.04.2021 22:10

Biology, 25.04.2021 22:10

History, 25.04.2021 22:10

Mathematics, 25.04.2021 22:10

Mathematics, 25.04.2021 22:10



Mathematics, 25.04.2021 22:10





Mathematics, 25.04.2021 22:10

History, 25.04.2021 22:10

Arts, 25.04.2021 22:10

History, 25.04.2021 22:10

Mathematics, 25.04.2021 22:10

Mathematics, 25.04.2021 22:10

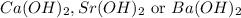
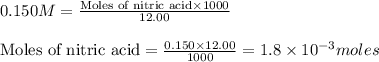

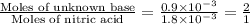
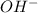 = 2 × number of
= 2 × number of 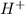 ions
ions

