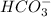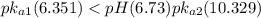Chemistry, 03.03.2020 03:40 josuemartinez1030
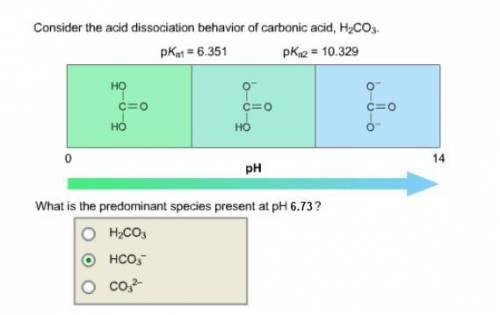
Consider the acid dissociation behavior of carbonic acid, H 2 CO 3 . A p H gradient from 0 to 14 is given. Below a p H equal to p K a 1 which is 6.351, the predominant form is H 2 C O 3. Above a p H equal to p K a 2 which is 10.329, the predominant form is C O 3 2 minus. Between the two p K a values, the predominant form is H C O 3 minus. What is the predominant species present at pH 6.73 ?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1
You know the right answer?
Consider the acid dissociation behavior of carbonic acid, H 2 CO 3 . A p H gradient from 0 to 14 is...
Questions



English, 07.01.2022 20:30

Mathematics, 07.01.2022 20:30




English, 07.01.2022 20:30


History, 07.01.2022 20:40

Social Studies, 07.01.2022 20:40

History, 07.01.2022 20:40

Biology, 07.01.2022 20:40




Mathematics, 07.01.2022 20:40


Mathematics, 07.01.2022 20:40

Law, 07.01.2022 20:40