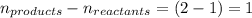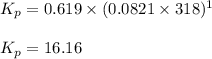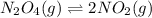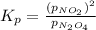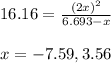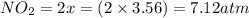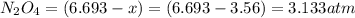Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
Part A If 50.0 gg of N2O4N2O4 is introduced into an empty 2.12 LL container, what are the partial pr...
Questions


Mathematics, 16.12.2021 20:40

Mathematics, 16.12.2021 20:40


Health, 16.12.2021 20:40

Geography, 16.12.2021 20:40

Mathematics, 16.12.2021 20:40


Chemistry, 16.12.2021 20:40


Biology, 16.12.2021 20:40

Mathematics, 16.12.2021 20:40



Mathematics, 16.12.2021 20:40





Health, 16.12.2021 20:40

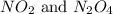 is 7.12 atm and 3.133 atm respectively.
is 7.12 atm and 3.133 atm respectively.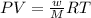
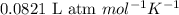
![45^oC=[45+273]K=318K](/tpl/images/0531/7110/fdbdd.png)
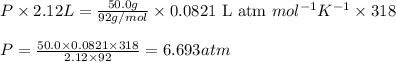
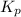 with
with 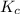 is given by the formula:
is given by the formula: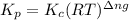
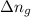 = change in number of moles of gas particles =
= change in number of moles of gas particles =