
Chemistry, 03.03.2020 03:56 s0cial0bessi0n
Carbon tetrachloride, CCl₄, is a solvent that was once used in large quantities in dry cleaning. Because it is a dense liquid that does not burn, it was also used in fire extinguishers. Unfortunately, its use was discontinued because it was found to be a carcinogen. It was manufactured by the following reaction:
The reaction was economical because the byproduct disulfur dichloride, S₂Cl₂, could be used by industry in the manufacture of rubber products and other materials.
a. What is the percentage yield of CCl₄i if 719 kg is produced from the reaction of 410. kg of CS₂?
b. If 67.5 g of Cl₂ are used in the reaction and 39.5 g of S₂Cl₂ is produced, what is the percentage yield?
c. If the percentage yield of the industrial process is 83.3%, how many kilograms of CS₂ should be reacted to obtain 5.00 × 10⁴ kg of CCl₄? How many kilograms of S₂Cl₂ will be produced, assuming the same yield for that product?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 23.06.2019 05:50
What is the molecular formula of ferrous nitrate and ferric nitrate
Answers: 2

Chemistry, 23.06.2019 07:30
How many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride
Answers: 1

Chemistry, 23.06.2019 11:30
Which part of the healthcare system could best explain how a pharmaceutical drug works
Answers: 2
You know the right answer?
Carbon tetrachloride, CCl₄, is a solvent that was once used in large quantities in dry cleaning. Bec...
Questions

Biology, 15.01.2021 03:40



Mathematics, 15.01.2021 03:40

Mathematics, 15.01.2021 03:40

Mathematics, 15.01.2021 03:40

Mathematics, 15.01.2021 03:40


Mathematics, 15.01.2021 03:40






Mathematics, 15.01.2021 03:40




English, 15.01.2021 03:40

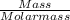 = 719/153.82 = 4.674 moles
= 719/153.82 = 4.674 moles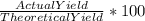 →
→ 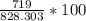 = 86.804 %
= 86.804 %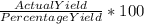 =
= 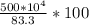 = 600.24 × 10⁴ kg
= 600.24 × 10⁴ kg

