
Chemistry, 03.03.2020 04:00 camiloriveraveoxbgd6
How many moles of sodium acetate must be added to 2.0 L of 0.10 M acetic acid to give a solution that has a pH equal to 5.00? Ignore the volume change due to the addition of sodium acetate.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 23.06.2019 02:30
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1
You know the right answer?
How many moles of sodium acetate must be added to 2.0 L of 0.10 M acetic acid to give a solution tha...
Questions

Business, 07.07.2021 21:10



Mathematics, 07.07.2021 21:10



Arts, 07.07.2021 21:10

Mathematics, 07.07.2021 21:10

Mathematics, 07.07.2021 21:10

Mathematics, 07.07.2021 21:10


Mathematics, 07.07.2021 21:10

Mathematics, 07.07.2021 21:10





Mathematics, 07.07.2021 21:10


Mathematics, 07.07.2021 21:10

![\frac{[acetate]}{[aceticacid]}](/tpl/images/0531/7594/7a095.png)
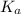 =
= 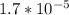
![\frac{[acetate]}{0.1}](/tpl/images/0531/7594/927c3.png)


