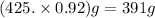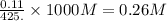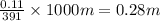A student dissolves 14.g of benzoic acid C7H6O2 in 425.mL of a solvent with a density of 0.92 g/mL. The student notices that the volume of the solvent does not change when the benzoic acid dissolves in it. Calculate the molarity and molality of the student's solution. Round both of your answers to 2 significant digits.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2

Chemistry, 23.06.2019 00:30
When did stem cell research become known ? who discovered stem cell? what experiments or studies have been conducted so far?
Answers: 3
You know the right answer?
A student dissolves 14.g of benzoic acid C7H6O2 in 425.mL of a solvent with a density of 0.92 g/mL....
Questions

History, 10.10.2019 15:30

Social Studies, 10.10.2019 15:30




World Languages, 10.10.2019 15:30




Mathematics, 10.10.2019 15:30


English, 10.10.2019 15:30

Mathematics, 10.10.2019 15:30




Mathematics, 10.10.2019 15:30

Mathematics, 10.10.2019 15:30


Mathematics, 10.10.2019 15:30

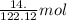 of benzoic acid = 0.11 mol of benzoic acid
of benzoic acid = 0.11 mol of benzoic acid