Chemistry, 03.03.2020 04:53 GhostElite6383
A 0.9679-g sample containing dimethylphthalate, (194.19 g/mol), and unreactive species was refluxed with 50.00 mL of 0.1215 M to hydrolyze the ester groups (this process is called saponification).
C6H4(COOCH3)2 + 2OH>> C6H4(COO)-2 + H2O
After the reaction was complete, the excess NaOH was back titrated with 32.25mL of 0.1251M HCl. Calculate the percentage of dimethylphthalate in the sample.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1


You know the right answer?
A 0.9679-g sample containing dimethylphthalate, (194.19 g/mol), and unreactive species was refluxed...
Questions



Mathematics, 15.01.2021 16:00


Mathematics, 15.01.2021 16:00

Mathematics, 15.01.2021 16:00


Health, 15.01.2021 16:00





Social Studies, 15.01.2021 16:00

Mathematics, 15.01.2021 16:00

Mathematics, 15.01.2021 16:10

Chemistry, 15.01.2021 16:10


English, 15.01.2021 16:10

Chemistry, 15.01.2021 16:10

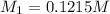
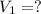

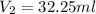
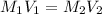
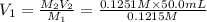
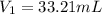
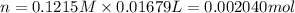

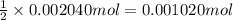 of dimethylphthalate
of dimethylphthalate