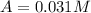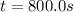Chemistry, 03.03.2020 06:07 Flowershere121
An average reaction rate is calculated as the change in the concentration of reactants or products over a period of time in the course of the reaction. An instantaneous reaction rate is the rate at a particular moment in the reaction and is usually determined graphically.
The reaction of compound A forming compound B was studied and the following data were collected:
Time (s) [A](M)
0. 0.184
200. 0.129
500. 0.069
800. 0.031
1200. 0.019
1500. 0.016
a.) What is the average reaction rate between 0. and 1500. s?
b.) What is the average reaction rate between 200. s and 1200. s?
c.) What is the instantaneous rate of the reaction at t=800 s?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:40
Does the energy in a solid increase or decrease when changing to a liquid?
Answers: 1

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2
You know the right answer?
An average reaction rate is calculated as the change in the concentration of reactants or products o...
Questions

Mathematics, 02.10.2020 17:01

Mathematics, 02.10.2020 17:01


Mathematics, 02.10.2020 17:01

History, 02.10.2020 17:01


English, 02.10.2020 17:01




Social Studies, 02.10.2020 17:01



History, 02.10.2020 17:01



Geography, 02.10.2020 17:01

English, 02.10.2020 17:01

Mathematics, 02.10.2020 17:01

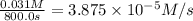
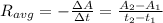
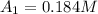
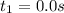
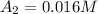
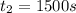
![R_{avg]=-\frac{0.016 M-0.184 M}{1500.0 s-0.0 s}=0.000112 M/s](/tpl/images/0531/9907/d3e1c.png)
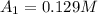
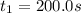
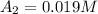
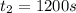
![R_{avg]=-\frac{0.019 M-0.129M}{1200.0s-200.0s}=0.00011 M/s](/tpl/images/0531/9907/ebcb3.png)