
Write the balanced net ionic equation for the reactions that occur when the given aqueous solutions are mixed. Include the physical states. A. silver nitrate, AgNO 3 AgNO3 , and magnesium bromide, MgBr 2 MgBr2 net ionic equation: B. perchloric acid, HClO 4 HClO4 , and potassium hydroxide, KOH KOH net ionic equation: C. ammonium sulfide, ( NH 4 ) 2 S (NH4)2S , and cobalt(II) chloride, CoCl 2 CoCl2 net ionic equation:

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2


Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1
You know the right answer?
Write the balanced net ionic equation for the reactions that occur when the given aqueous solutions...
Questions

Chemistry, 24.11.2020 20:40


Chemistry, 24.11.2020 20:40

Chemistry, 24.11.2020 20:40

History, 24.11.2020 20:40


Mathematics, 24.11.2020 20:40



Biology, 24.11.2020 20:40


Computers and Technology, 24.11.2020 20:40


Mathematics, 24.11.2020 20:40

History, 24.11.2020 20:40

Social Studies, 24.11.2020 20:40


Mathematics, 24.11.2020 20:40

Mathematics, 24.11.2020 20:40

English, 24.11.2020 20:40

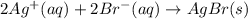
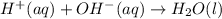
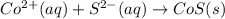
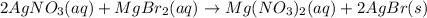
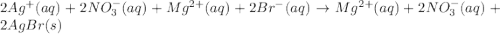
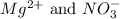 are the spectator ions.
are the spectator ions.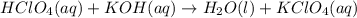
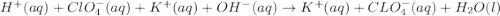
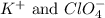 are the spectator ions.
are the spectator ions.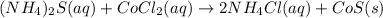
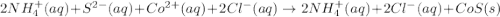
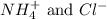 are the spectator ions.
are the spectator ions.


