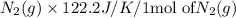Chemistry, 03.03.2020 06:05 Frenchfries13
Consider the reaction: N2(g) + 2O2(g)2NO2(g) Using standard absolute entropies at 298K, calculate the entropy change for the system when 1.90 moles of N2(g) react at standard conditions. S°system = J/K Submit Answer

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1


Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3
You know the right answer?
Consider the reaction: N2(g) + 2O2(g)2NO2(g) Using standard absolute entropies at 298K, calculate th...
Questions



Mathematics, 20.08.2019 16:50



Mathematics, 20.08.2019 16:50





Chemistry, 20.08.2019 16:50


Geography, 20.08.2019 16:50

History, 20.08.2019 16:50


Mathematics, 20.08.2019 16:50


Mathematics, 20.08.2019 16:50


Social Studies, 20.08.2019 16:50

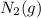 is 191.6 J/mol K,
is 191.6 J/mol K, 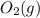 = 205 J/mol K, and
= 205 J/mol K, and 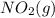 is 239.7 J/mol K at 298 K.
is 239.7 J/mol K at 298 K.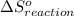 from standard absolute entropies as follows.
from standard absolute entropies as follows.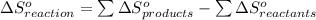
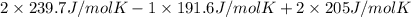
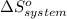 = 1.90 moles of
= 1.90 moles of