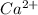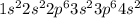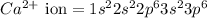Chemistry, 03.03.2020 19:51 Michael845313
The following Lewis diagram represents the valence electron configuration of a main-group element. This element is in group 2A According to the octet rule, this element would be expected to form a(n) with a charge of cation anion If X is in period 4, the ion formed has the same electron configuration as the noble gas The symbol for the ion is

Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
The following Lewis diagram represents the valence electron configuration of a main-group element. T...
Questions

Biology, 05.01.2020 23:31


Mathematics, 05.01.2020 23:31

Mathematics, 05.01.2020 23:31

English, 05.01.2020 23:31






Mathematics, 05.01.2020 23:31




Mathematics, 05.01.2020 23:31


Chemistry, 05.01.2020 23:31

English, 05.01.2020 23:31


Social Studies, 05.01.2020 23:31